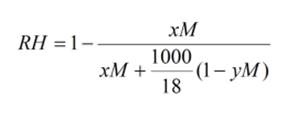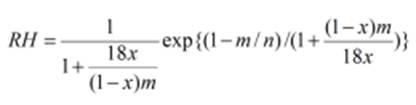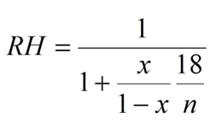![]()
|
Crystal Dehydration |
|
|
The EMBL HC1
Humidity Control device allows the modulation of the diffraction
properties of crystals by controlled dehydration. The first step in a
dehydration experiment is to define the relative humidity in equilibrium with
the mother liquor of the system under study; this can often be quite
time-consuming. In order to reduce the time spent on this stage of the
experiment, the equilibrium relative humidity for a range of concentrations
of the most commonly used precipitants has been measured. The relationship
between the precipitant solution and equilibrium relative humidity is
explained by Raoult's law for the equilibrium
vapour pressure of water above a solution. The concentration of buffers,
additives and detergents used will have a negligible effect on the RH in
equilibrium with the mother liquor and is dominated by the primary
precipitant. Use
the applet below to predict the RH in equilibrium with your mother liquor
before the experiment. Once here the value can be refined using the HC1. Contact
Matthew Bowler for
help. Please cite Wheeler,
M.J., et al. (2012) Acta Cryst. F68, 111-114 if you use these equations to
calculate the RH in equilibrium with solutions or for starting points for
experiments using the HC1 humidity control device. The Relative Humidity
(RH) in equilibrium with solutions can be understood in terms of Raoult's law. It has two aspects that are
counter-intuitive and lead to some surprising observations. The first is that
the number of equivalent molecules in solution must be accounted for. This
means that for sodium chloride, each ion in solution counts as a molecular
equivalent. This requires knowledge of the ionization behaviour of the
substance in solution. For example, ammonium sulfate
effectively dissociates into two ions [NH4+ and (NH4SO4)-]
and not three as might be expected. Raoult's
law starts to break down for PEG solutions over a molecular weight of 1000 Da
but this can be corrected using the Flory-Huggins model for the entropy of
mixing (used in equation 2).
Equation
1: This equation will enable you to calculate the Relative
Humidity in equilibrium with a salt solution. You will need to provide the
concentration in Mol, the ionisation state of the
salt (how many species it dissociates into) and a term describing the the specific volume of the solute (for sodium chloride y
= 0.027, for ammonium sulfate y = 0.074, for sodium
acetate y = 0.054, for sodium malonate y = 0.095,
for magnesium sulphate y = 0.045, for Monopotassium
phosphate y = 0.058 and for Dipotassium phosphate y
= 0.071). Raoult's law breaks down for lithium
chloride (y=0.023) concentrations over 2.5M with RH values much lower than
predicted.
Equation 2: where RH is the relative humidity, x is the mass fraction of
solute, n is the molecular weight of the polymer and m is a parameter for a
polymer such that the ratio n/m is the number of segments of polymer, each of
which takes up one space in the disordered lattice. We have found that a
value of 38 Da gives a best fit to experimental rather than the actual value
of 44 Da for PEGs. Thus, m is approximately the molecular weight of the
monomer, when each of the segments is a monomer. For n>>m the
dependence on n diminishes as a function of the mass fraction x, so the
relative humidity curve has a very nearly universal shape for polymers of
molecular mass above ca. 1000 Da. Therefore, the RH equilibrium points for
all PEGs of >1000 Da will be equal.
Equation 3: This
equation will allow you to calculate the Relative Humidity (RH) in
equilibrium with solutions of substances that neither dissociate into
multiple species nor are polymers (e.g glycerol,
ethylene glycol, sucrose, TMAO, etc) |