![]()
|
Crystal Dehydration |
|
|
Humidity Control Device (HC1)
Overview Controlled dehydration of protein crystals has often been reported as a key factor to improve the diffraction quality of the crystals. Increases in resolution limit, decreases in the mosaicity and space-group transitions are well documented (Kaushal et al. 2008, Bowler et al. 2006, Kuo et al. 2003, Saraswathi et al. 2002) and different approaches have been developed to carry out the dehydration process (Heras & Martin, 2005, Kiefersauer et al. 2000,).
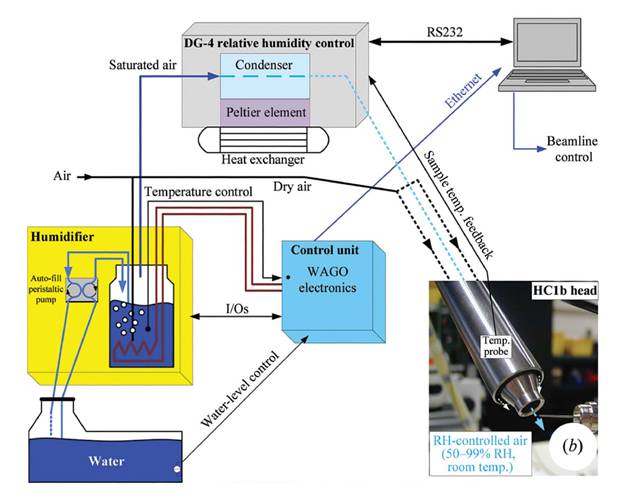
The Humidity Control device (HC1), developed by the EMBL Grenoble Diffraction Instrumentation Team and based on simple but robust technology was designed to be user friendly and completely adapted to MX beamlines.
Schematic of the HC1
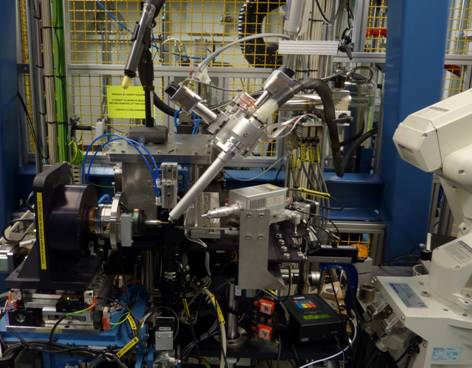
The HC1 is available at several synchrotrons facilities, including the ESRF (France), DLS (UK), Max-lab (Sweden) and CLS (Canada).
Setup of the HC1 @ ESRF and DLS MX beamlines
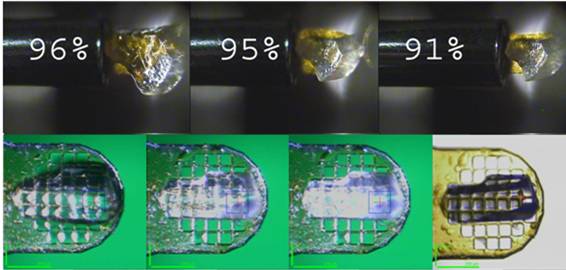
Using the device Crystals are mounted on meshes (from Mitegen or MDL) and kept in the humid air stream from a modified cryostream nozzle. The first step in a dehydration experiment is to define the relative humidity in equilibrium with the mother liquor of the system under study. Use this form to calculate the RH in equilibrium with your precipitant. Once crystals have been conditioned at the optimum RH level they can be immediately cryocooled by simply unmounting them with the sample changer. The operation of the HC1 is fully described at the HC1 User’s Manual.
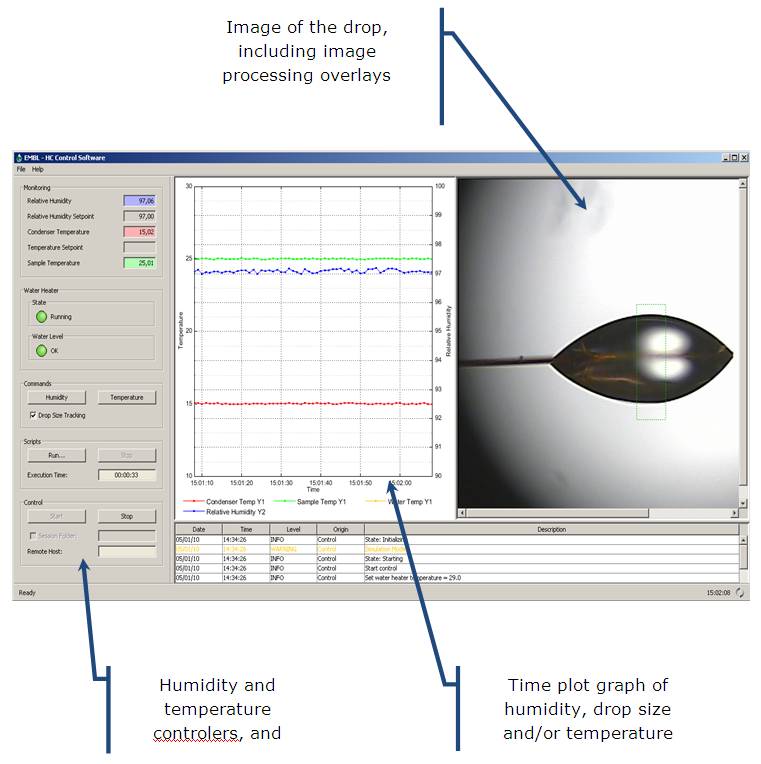
Software interface
For
a detailed description of the software please see HC1 Software’s Manual.
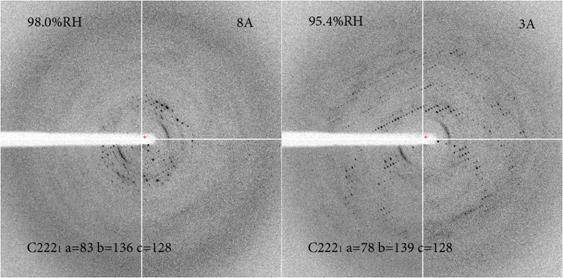
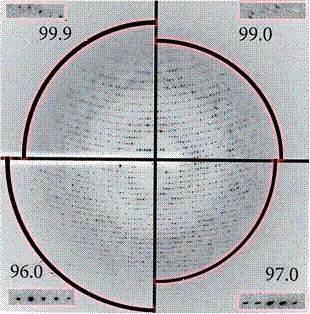
Successful experiments Several systems have benefited from the device and some of the examples are fully described in Sanchez-Weatherby et al. (2009) Acta Cryst. D65, 1237-1246 and Russi et al. (2011) J. Struct. Biol. 175, 236-243 (please, cite these references in any paper that reports or involved experiments performed with the HC1).
Chromatin-modification complex
F1-ATPase
|